SATIN
Safety of TKI concurrent with cranial radiotherapy in NSCLC patients; the SATIN platform trial
Enrollment
Recruiting
No. of patients
4 / 140
Population
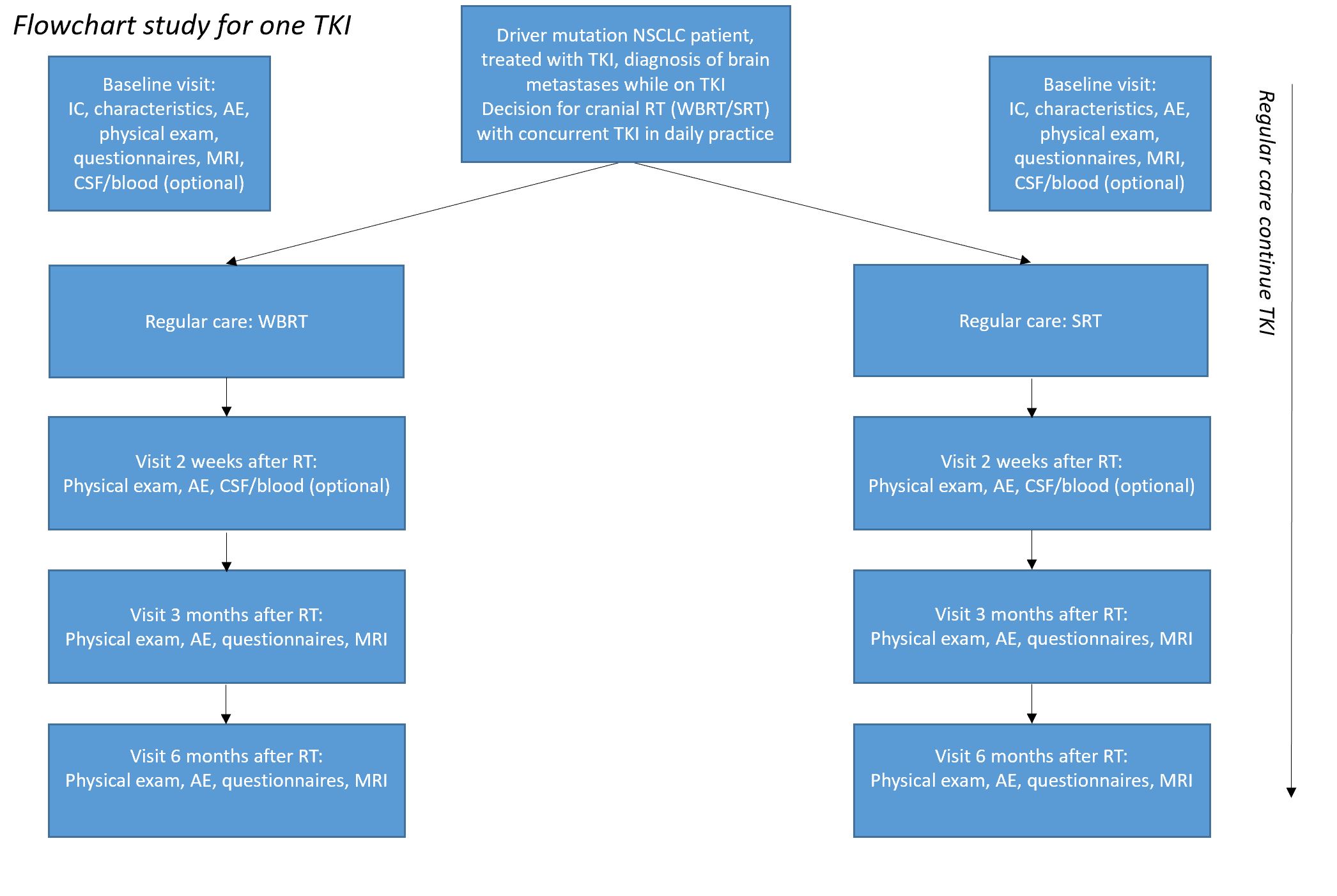
Patients with stage IV NSCLC with a driver mutation, who developed brain metastases during TKI treatment and are planned for WBRT or SRT (as part of standard care) with continuation of their TKI
Design
An observational, multicenter study. There will be different cohorts, every TKI will be assessed separately. (erlotinib, gefitinib, afatinib, osimertinib, crizotinib, ceritinib, alectinib). For every TKI there is a WBRT (N=10) and a SRT (N=10) cohort
Key Outcome parameters
Primary endpoints:
- Increase in incidence of acute severe toxicity (per TKI) 2 weeks after completion of cranial
- Radiotherapy, measured with CTCAE v 4.0, compared to baseline data
- Increase in neurotoxicity 4 months after completion of cranial radiotherapy compared to baseline data
Secondary endpoints:
- Increase in neurotoxicity 6 months after completion of cranial radiotherapy compared to baseline data
Exploratory endpoints:
- Intracranial PFS at 4 and at 6 months after completion of cranial radiotherapy (measured with MRI)
- Cerebrospinal fluid penetration potential, measured by the unbound cerebrospinal fluid-to-plasma ratio (Kp,uu), before and 2 weeks after completion of cranial radiotherapy (optional for patients) and to correlate this ratio with toxicity (CTCAE v 4.0) 2 weeks after cranial radiotherapy (optional)
- Plasma concentration of the TKI before and 2 weeks after cranial radiotherapy and to correlate this concentration with toxicity (CTCAE v 4.0) 2 weeks after cranial radiotherapy
Intervention
Standardized neurocognitive examination will be done before radiotherapy, after 4 months and after 6 months. A magnetic resonance imaging (MRI) of the brain will be performed at 4 and at 6 months (partly already usual care). Optionally cerebrospinal fluid (CSF) will be obtained by lumbar puncture before and two weeks after radiotherapy. Optionally blood will be obtained by venepuncture before and two weeks after radiotherapy
Key inclusion criteria
Inclusion criteria
- Stage IV NSCLC with driver mutation, treated with TKI
- Development of brain metastases during TKI treatment
- Indication for cranial radiotherapy determined by treating physician and radiation oncologist with continuation of the TKI
- Age ≥ 18 years
- Ability to understand neurocognitive testing
- Written informed consent
Key exclusion criteria
Exclusion criteria
- Prior radiotherapy to the brain when this precludes new radiotherapy.
- Neurologic/psychiatric illnesses (such as Alzheimer’s disease)
- Claustrophobia
- Metal implants or other contra-indication for MRI
- Inability to lie supine for 30 minutes time (MRI)
- Pregnancy
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.