PAULIEN
Pembrolizumab alone versus pembrolizumab-chemotherapy in first line NSCLC
Enrollment
Recruiting
No. of patients
62 / 84
Population
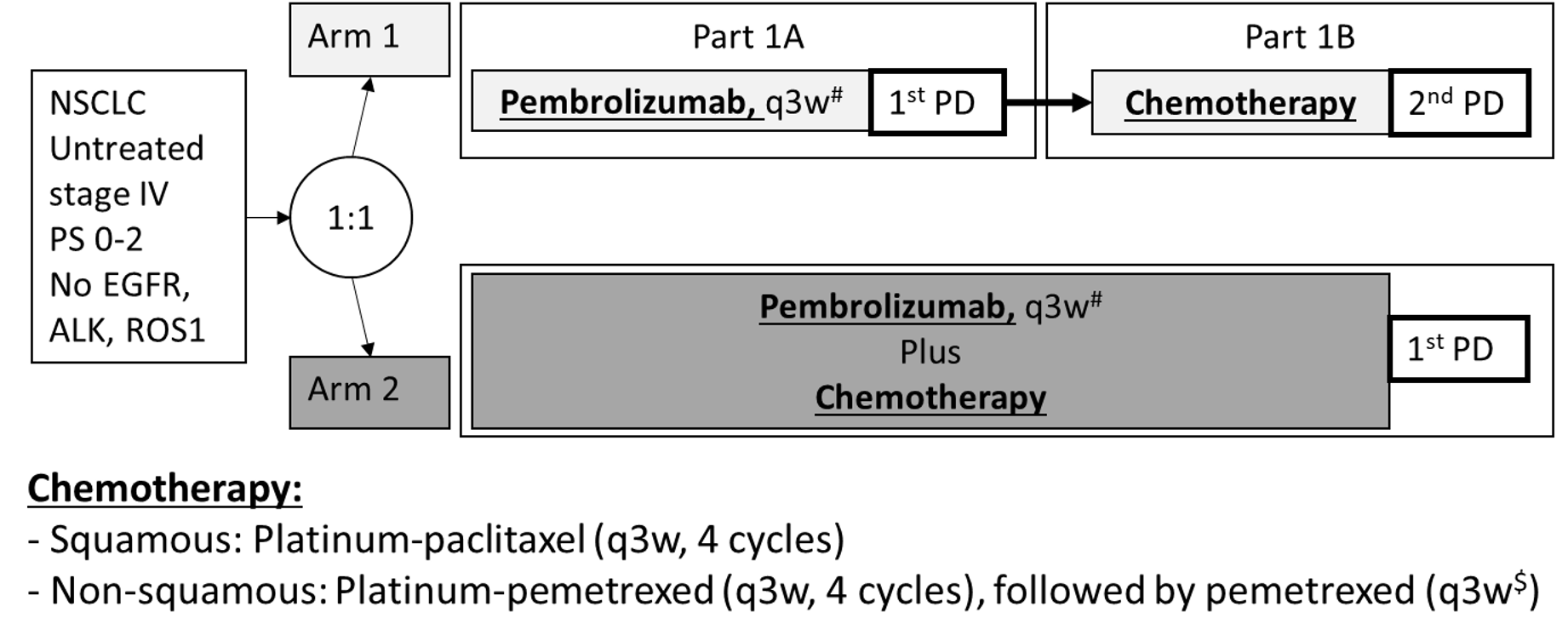
Stage 4 NSCLC, with TPS≥50%, and at least a cTxNxM1c
Design
An open label, phase 3, randomized clinical trial
Key outcome parameters
Primary endpoints:
- ORR, as defined by partial response (PR) and complete response (CR) at week 6
- Disease control rate (DCR), as defined by stable disease (SD) and PR and CR at week 6
Secondary endpoints:
- PFS, as determined using Response Evaluation Criteria in Solid Tumors (RECIST1.1) and defined as time to the development of new lesions, progression of existing lesions, or death, whichever comes first
- PFS-2, as defined by the PFS on second line chemotherapy in arm 1
- ORR-2, as defined by the ORR on second line chemotherapy in arm 1
- Overall Survival (OS). Time frame: Baseline until death
- Safety defined as the percentage of patients with adverse events. Adverse events will be defined as described in the Common Terminology Criteria for Adverse Events (CTCAE)
Intervention
Arm 1: pembrolizumab alone (200mg fixed dose, 3 weekly) until progressive disease (PD)
Arm 2: pembrolizumab (200mg fixed dose, 3 weekly) with chemotherapy (carboplatin AUC 5 or cisplatin (75 or 80mg/m2) combined with either pemetrexed (500mg/m2, non-squamous) or paclitaxel (200mg/m2, squamous). Chemotherapy doublets will be given for 2-4 cycles depending on the tumor response, also, pemetrexed and pembrolizumab will continued as maintenance until PD or unacceptable toxicity in arm 2
Key inclusion criteria
- Histologically confirmed NSCLC, negative for EGFR mutations and ALK fusions, no molecular testing is required in squamous NSCLC
- ECOG Performance Scale 0-2
- Be willing and able to provide written informed consent for the trial
- Be 18 years or older of age on the day of signing informed consent
- Have measurable disease based on RECIST v1.1
- Must provide tissue from a histological tumor biopsy that was not yet irradiated
- High tumor PD-L-1 expression (≥50% TPS)
- High tumor burden (≥2 extrapulmonary metastases (M1c)) and not amenable for local consolidative therapies
- Must have adequate hematologic and organ function
Key exclusion criteria
- Patients amenable for local consolidative therapies
- Use of steroids equivalent to >10 mg prednisolon per day prior to start of study or other immunosuppressive medications within 14 days prior. Inhaled or topical steroids, and adrenal replacement steroid >10 mg daily prednisone equivalent, are permitted in the absence of active autoimmune disease
- Untreated brain metastases
- Uncontrolled active infections, HIV, active Hepatitis B or C
- Autoimmune diseases and interstitial lung diseases are to be excluded depending on physicians decision
- A known additional malignancy that is progressing or requires active treatment. Exceptions include basal cell carcinoma of the skin, squamous cell carcinoma of the skin, or in situ cervical cancer that has undergone potentially curative therapy
- Known psychiatric or substance abuse disorders that would interfere with cooperation with the requirements of the trial
- Is pregnant or breastfeeding, or expecting to conceive children within the projected duration of the trial, starting with the screening
- Prior systemic therapy for the NSCLC using chemotherapy or immunotherapy with prior therapy with an anti-PD-1, anti-PD-L1, anti-PD-L2, anti-CTLA-4 antibody, or any other antibody or drug specifically targeting T-cell co-stimulation or immune checkpoint pathways
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.