ADAPT/ALEC
A prospective randomized controlled trial comparing standard treatment with ALK inhibitor alectinib to dose adjusted alectinib based on therapeutic drug monitoring
Enrollment
Recruiting
No. of patients
3 / 196
Population
Patients with locally advanced or metastatic NSCLC (stage IIIB to stage IV)
Design
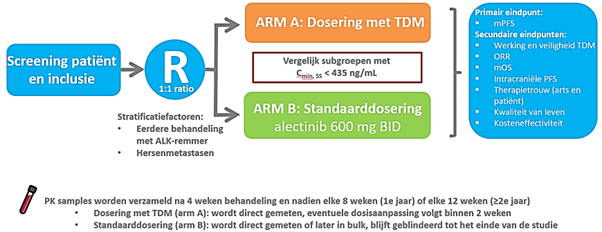
A prospective randomized controlled trial comparing standard treatment with ALK inhibitor alectinib to dose adjusted alectinib based on therapeutic drug monitoring. Patients will be stratified based on prior treatment with other ALK inhibitors and brain metastases.
Key outcome parameters
Primary endpoints:
- The primary objective will be a prolonged mPFS in the TDM-guided dosing arm for the subgroup who had a Cmin < 435 ng/mL at a certain time point during treatment, compared to these patients in the fixed dosing arm.
Secondary endpoints:
- Feasibility and safety of TDM
- Physician adherence to TDM advice
- Overall response rates (ORR)
- Median overall survival (mOS)
- Intracranial PFS
- Patient adherence
- Toxicity (also in relationship to alectinib plasma concentrations and dose increases).
Intervention
Therapeutic Drug Monitoring
Key inclusion criteria
Inclusion criteria:
- Histologically or cytology confirmed NSCLC
- Documented ALK rearrangement based on an EMA approved test
- Measurable disease (by RECIST criteria version 1.1) prior to the first dose of study treatment
- Male or female ≥18 years old
- ECOG Performance Status of 0−4
- Local radiotherapy is allowed for pain
Key exclusion criteria
Exclusion criteria:
- Any significant concomitant disease determined by the investigator to be potentially aggravated by the investigational drug
- Consumption of agents which modulate CYP3A4 or agents with potential QT prolonging effects within 14 days prior to admission and during the study (see concomitant medication restrictions)
- Any clinically significant concomitant disease or condition that could interfere with, or for which the treatment might interfere with, the conduct of the study, or absorption of oral medications, or that would, in the opinion of the Principal Investigator, pose an unacceptable risk to the subject in this study
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol requirements and/or follow-up procedures; those conditions should be discussed with the patient before trial entry
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.