ITHACA
Immune therapy associated cardiovascular complications (ITHACA)
A clinical imaging study on cardiovascular complications of immune checkpoint inhibitors (ICI) using coronary angiography to investigate plaque progression after initiation of ICI
Enrollment
Recruiting
No. of patients
13/214
Population
Patients with non-small-cell lung carcinoma planned for pembrolizumab (high PD-L1 expression based on TPS ≥50%, stage IV) or durvalumab (stage III);
Controls: patients with non-small-cell lung carcinoma planned for other systemic therapy than ICI
Design
A single center, prospective observational study
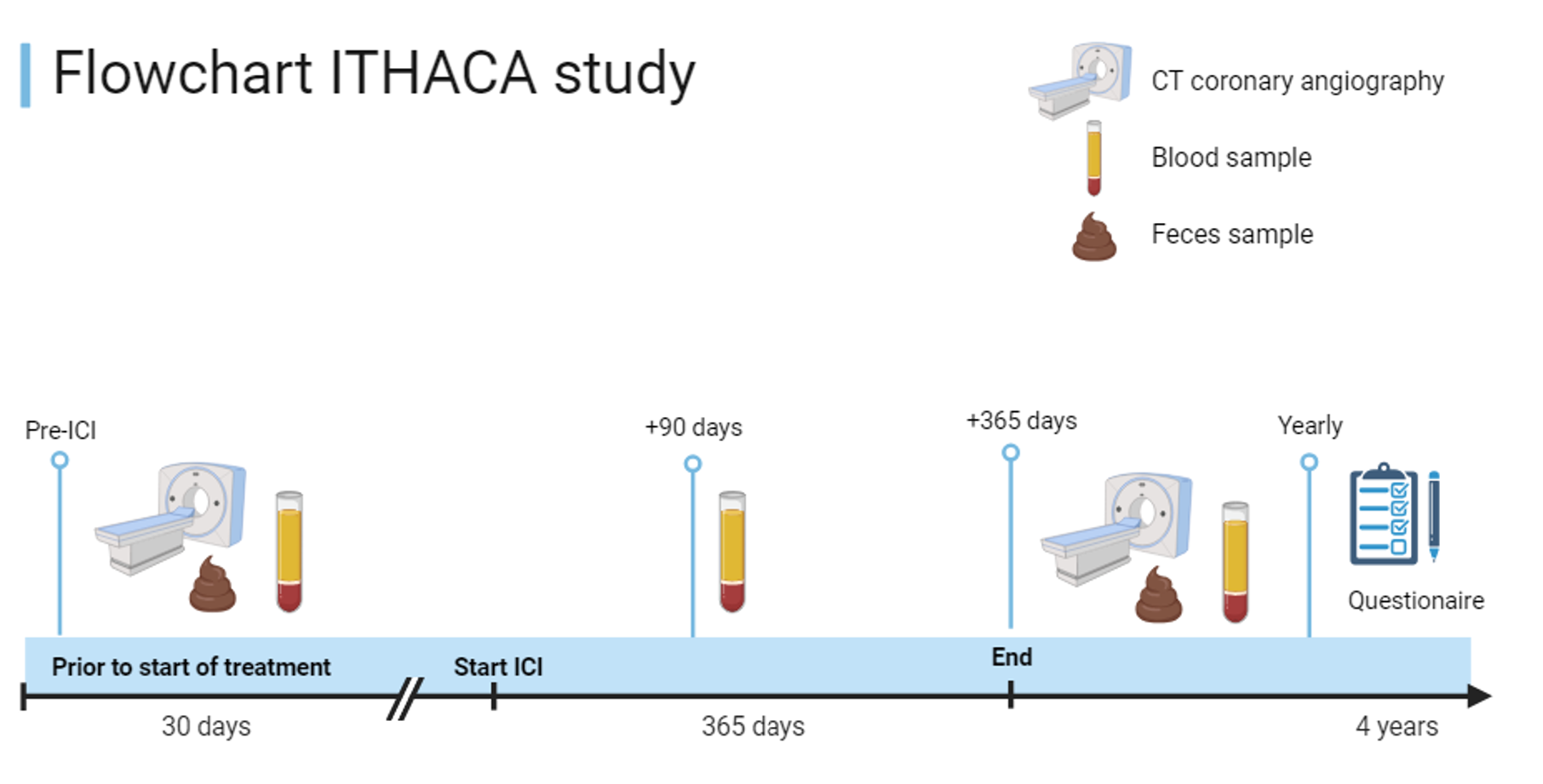
Key outcome parameters
Primary endpoints:
- Difference in medion non-calcified coronary plaque volume between baseline and 1-year follow-up on computed tomography angiography (CCTA)
Secondary endpoints:
- Atherosclerotic plaque changes (i.e. fatenuation index, non-calcified plaques);
- Plasma and fecal biomarker changes associated with immunothrombosis;
- Incidence of arterial and venous thromboembolic events;
- Incidence of immune related adverse events (irAE)
Intervention
All patients will undergo CCTA scanning at baseline and after 1 year. Blood will be drawn at baseline, 3 and 12 months after start of therapy. Feces will be collected at baseline and after 1 year.
Key inclusion criteria
Inclusion criteria:
- Confirmed diagnosis of non-small-cell lung carcinoma planned for pembrolizumab (high PD-L1 expression based on TPS ≥50%, stage IV), durvalumab (stage III) or other than ICI (controls);
- Age ≥50 years;
- Prior to start of new therapy
Key exclusion criteria
Exclusion criteria:
- ICI therapy in previous 12 months;
- Suspected or confirmed viral, fungal, or bacterial infectious disease;
- Use of immunosuppressive therapy prior to ICI start;
- Estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2;
- Known allergy to iodinated contrast agents;
- Atrial fibrillation.
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.