TRIDENT-1 (AvL)
A Phase 1/2, Open-Label, Multi-Center, First-in-Human Study of the Safety, Tolerability, Pharmacokinetics, and Anti-Tumor Activity of TPX-0005 (Repotrectinib) in Patients With Advanced Solid Tumors Harboring ROS1, or NTRK1-3 Rearrangements
Enrollment
Phase 1 = closed
Phase 2 = recruiting
No. of patients
00 / 00
Population
Adult patients with confirmed diagnosis of locally advanced, or metastatic solid tumor (including primary CNS tumors) (Stage IV) that harbors an ALK, ROS1, NTRK1, NTRK2, or NTRK3 gene fusion
Design
Phase 1/2 open-label, multicenter study
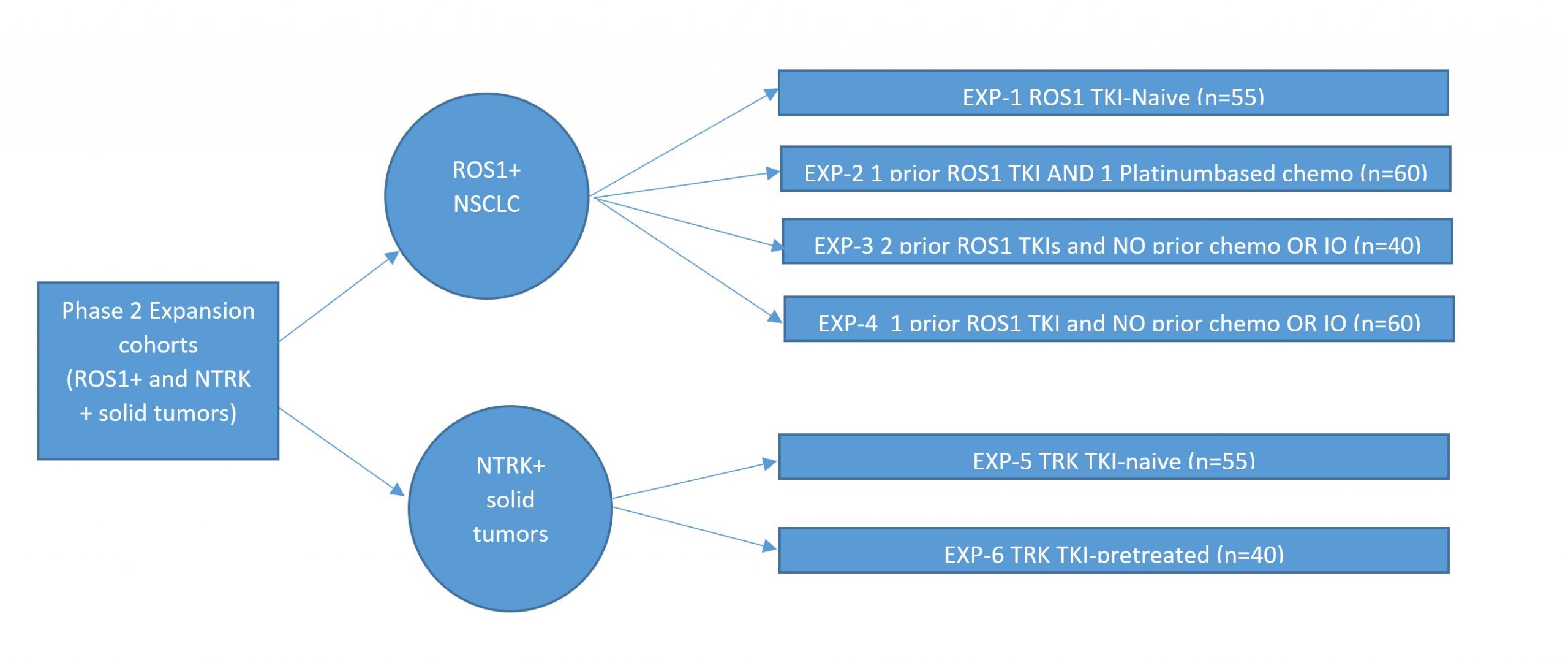
Key outcome parameters
Primary endpoints:
- To determine the confirmed Objective Response Rate (ORR) as assessed by Blinded Independent Central Review (BICR) of repotrectinib in each subject population expansion cohort of advanced solid tumors that harbor a ROS1, NTRK1, NTRK2, or NTRK3 gene rearrangement
Secondary endpoints:
- To determine the Duration of Response (DOR), time to response (TTR), and clinical benefit rate (CBR) of repotrectinib, as assessed by BICR, in each subject population expansion cohort of advanced solid tumors that harbor a ROS1, NTRK1, NTRK2, or NTRK3 gene rearrangement
- To estimate the progression-free survival (PFS) and overall survival (OS) of subjects treated with repotrectinib with advanced solid tumors that harbor a ROS1, NTRK1, NTRK2, or NTRK3 gene rearrangement
- To evaluate the safety and tolerability of repotrectinib when administered at the RP2D in subjects with advanced solid tumors that harbor a ROS1, NTRK1, NTRK2, or NTRK3 gene rearrangement
- To determine the intracranial objective response rate (IC-ORR) of repotrectinib and Central Nervous System PFS (CNS-PFS) in subjects presenting with measurable brain metastases at baseline, using modified Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 assessment
- To confirm PK of repotrectinib at the RP2D
Intervention
Repotrectinib 160mg BID
Key inclusion criteria
Inclusion criteria
- Histologically or cytologically confirmed diagnosis of locally advanced, or metastatic solid tumor (including primary CNS tumors) that harbors a ROS1 or NTRK1-3 gene fusion. Note: Locally advanced disease is defined as Stage III when patient is not a candidate for surgery, radiation, or multi-modality therapy and metastatic disease is defined as Stage IV per the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual guidelines
- Subject must have a documented ROS1 or NTRK1-3 gene fusion determined by tissue-based local testing using either:
- a) a next-generation sequencing (NGS) or quantitative polymerase chain reaction (qPCR) test will be accepted to determine molecular eligibility
- Adequate tumor tissue needs to be sent to the Sponsor designated central diagnostic laboratory for retrospective confirmation by a central diagnostic laboratory test selected by the Sponsor. In cases where archived tumor tissue is not available, a de novo biopsy should be obtained at Screening or as soon as possible after enrollment. See the Study Laboratory Manual for details
- If NGS was used, the partner of the fusion target gene needs to be identified
- Retrospective confirmation by a central diagnostic laboratory test selected by the Sponsor is not required if ROS1 or NTRK1-3 gene fusion were determined by the Repotrectinib clinical trial assay (CTA)
- a) a next-generation sequencing (NGS) or quantitative polymerase chain reaction (qPCR) test will be accepted to determine molecular eligibility
- Or:
- b) a fluorescence in situ hybridization (FISH) test AND prospective confirmation of fusion status by a central diagnostic laboratory test selected by the Sponsor PRIOR to enrollment will be accepted to determine molecular eligibility
- Adequate tumor tissue must be sent to the Sponsor designated central diagnostic laboratory for prospective confirmation by a central diagnostic laboratory test selected by the Sponsor PRIOR to enrollment. In cases where archived tumor tissue is not available, a de novo biopsy should be obtained for this purpose
- A t least 1 measurable target lesion according to RECIST (v1.1) prospectively confirmed by Blinded Independent Central Radiology Review (BICR), selected by the Sponsor, PRIOR to enrollment. Subjects with CNS-only measurable target lesion ≥ 10 mm as defined by RECIST (v1.1) are eligible
- b) a fluorescence in situ hybridization (FISH) test AND prospective confirmation of fusion status by a central diagnostic laboratory test selected by the Sponsor PRIOR to enrollment will be accepted to determine molecular eligibility
Key exclusion criteria
Exclusion criteria
- Concurrent participation in another therapeutic clinical trial
- Symptomatic brain metastases or leptomeningeal involvement
- History of previous cancer requiring therapy within the previous 2 years, except for squamous cell or basal-cell carcinoma of the skin, or any in situ carcinoma that has been completely resected
- Major surgery within 4 weeks of start of repotrectinib treatment. Radiation therapy (except palliative to relieve bone pain) within 2 weeks of study entry. Palliative radiation (≤ 10 fractions) must have been completed at least 48 hours prior to study entry
- Clinically significant cardiovascular disease (either active or within 6 months prior to enrollment): myocardial infarction, unstable angina, coronary/peripheral artery bypass graft, symptomatic congestive heart failure (New York Heart Association Classification Class ≥ II), cerebrovascular accident or transient ischemic attack, symptomatic bradycardia, requirement for anti-arrhythmic medication. Ongoing cardiac dysrhythmias of CTCAE grade ≥ 2
Contact opnemen over een studie
Neem contact op voor meer informatie over de studies van de afdeling thoracale oncologie van Amsterdam UMC.